Introduction to Medical Device Registration in India
In India, the registration system for medical devices is a critical step to make certain their safety, efficacy, and first-class. It is ruled via regulatory bodies that set standards and suggestions to guard public fitness. Understanding the intricacies of this system is essential for producers aiming to introduce their merchandise into the Indian market.
Table of Contents
Toggle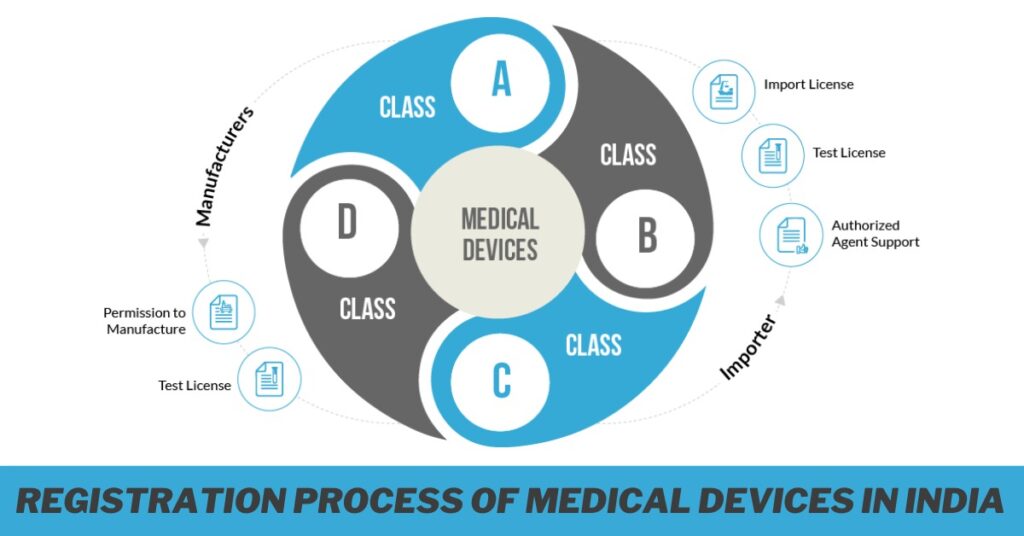
Registration Process of Medical Devices Regulatory Framework
The regulatory framework for scientific gadgets in India is complete and is continuously evolving to maintain tempo with technological advancements and global standards. The primary regulation governing clinical tool registration is the Drugs and Cosmetics Act, 1940, and the Medical Device Rules, 2017. These laws define the necessities for the import, manufacture, and sale of medical devices inside the country.
Classification of Medical Devices
Medical devices are labeled into different classes based totally on their hazard stage and supposed use. The category criteria include factors such as the duration of contact with the frame, invasiveness, and capability harm to the patient. The type determines the regulatory pathway and the documentation required for registration.
Preparation for Registration
Before beginning the registration process, manufacturers want to make sure that they’ve all the necessary documentation in location. This consists of technical documents, along with product specs, production techniques, and high-quality manage methods. Establishing a strong great management device is likewise crucial to conform with regulatory necessities.
Medical Devices in India Application Process
The utility for medical device registration is submitted to the Central Drugs Standard Control Organization (CDSCO) together with the specified files and fees. The regulatory authorities then evaluate the application based on elements which include protection, efficacy, and great. This assessment process may additionally contain scrutiny of scientific records and product checking out.
Medical Devices in India Clinical Trials and Testing
Clinical trials play a essential function in demonstrating the safety and efficacy of scientific gadgets. Depending on the danger class, manufacturers may be required to behavior clinical trials in India to generate nearby medical statistics. Additionally, trying out of the tool in line with applicable Indian or worldwide requirements is crucial to assess its overall performance.
Medical Devices in India Labeling and Packaging
Regulations governing the labeling and packaging of medical gadgets aim to offer essential records to healthcare professionals and sufferers. Labels ought to consist of information together with the product name, manufacturer’s data, meant use, and commands to be used. Proper packaging is likewise vital to protect the device in the course of garage and transportation.
Post-Market Surveillance
Even after acquiring registration, producers are required to screen the performance in their medical gadgets inside the market. Post-market surveillance entails amassing and analyzing records on detrimental events, product lawsuits, and device disasters. This information allows regulatory authorities identify capability risks and take suitable movement to mitigate them.
Renewal and Amendments
Registrations for medical devices in India are typically legitimate for a targeted length, and then they want to be renewed. Manufacturers also are required to tell the regulatory government approximately any adjustments or changes to the device or its manufacturing technique. This guarantees that the tool maintains to satisfy regulatory requirements for the duration of its lifecycle.
Challenges and Roadblocks
Navigating the registration method for clinical devices in India may be tough because of various factors which includes complicated rules, lengthy approval timelines, and the want for local medical facts. Manufacturers may additionally face hurdles in acquiring regulatory clearance, that could postpone market entry. However, proactive making plans and strategic partnerships can assist conquer these limitations.
Importance of Compliance
Compliance with regulatory necessities is paramount for manufacturers to advantage market get admission to and preserve purchaser agree with. Adhering to nice and protection standards no longer most effective ensures the reliability of scientific devices but also protects public health. Non-compliance can lead to consequences, product recollects, and damage to the organisation’s popularity.
Recent Developments
In current years, there have been giant traits in India’s regulatory landscape for clinical devices. The authorities has introduced projects to streamline the registration system, decorate post-marketplace surveillance, and align with worldwide requirements. These trends aim to foster innovation whilst ensuring patient protection and access to great healthcare.
Future Outlook
The future of scientific device registration in India is anticipated to be shaped by using advancements in generation, adjustments in healthcare regulations, and global regulatory traits. There is a developing emphasis on harmonizing policies with international standards to facilitate trade and sell innovation. Manufacturers ought to live updated on regulatory trends and adapt their strategies thus.
Conclusion
The registration process for clinical devices in India is a multifaceted journey that calls for careful making plans, compliance with regulations, and ongoing dedication to nice. By information the regulatory framework, making ready thorough documentation, and addressing demanding situations proactively, producers can navigate the system efficiently and contribute to enhancing healthcare results inside the us of a.
FAQs (Frequently Asked Questions)
- What is the common timeline for medical device registration in India?
- The timeline for medical tool registration in India can vary depending on factors including the complexity of the tool, the completeness of the application, and regulatory assessment instances. On common, the technique might also take anywhere from numerous months to a few years.
- Can overseas manufacturers observe for registration in India?
- Yes, foreign producers can follow for registration of their scientific gadgets in India. However, they ought to employ a licensed agent or importer in India who might be liable for regulatory compliance and communication with the government.
- Are there any unique requirements for software program-based scientific devices?
- Yes, software-based totally scientific gadgets are situation to precise requirements mentioned via regulatory authorities in India. These may additionally encompass documentation related to software validation, cybersecurity, and data privateness.
- What function does the Central Drugs Standard Control Organization (CDSCO) play inside the registration procedure?
- The CDSCO is the primary regulatory authority accountable for overseeing the registration and regulation of clinical gadgets in India. It evaluates packages, conducts inspections, and video display units put up-marketplace surveillance activities to ensure compliance with regulatory requirements.
- How often do regulations governing scientific device registration in India get updated?
- Regulatory requirements for scientific tool registration in India are problem to periodic updates to align with international requirements and address emerging demanding situations. Manufacturers have to stay knowledgeable about these changes to ensure compliance with the modern policies.

