In India, all medical devices and in-vitro diagnostic devices are regulated under Medical Device Rule, 2017. All medical devices and in-vitro diagnostic devices are classified into four categories named A, B, C, and D depending on the level of risk associated with the devices. Those companies who want to manufacture medical devices in India must have a medical device manufacturing license from the licensing authority.
State Licensing Authority is responsible for the grant of manufacturing licenses for Class A and B devices. Central Licensing Authority CDSCO is responsible for the grant of manufacturing licenses for Class C & D. If you want to manufacture Class A and Class B devices, then you must get the Medical Device Manufacturing License on Form MD 5 from the State Licensing Authority.
We at MedDev Experts, guide our clients on how to comply with the Medical Device Rule, 2017 and get MD 5 License to Manufacture Class A or B Medical Devices and IVDs. Our highly experiences regulatory experts will guide you throughout the process from gap analysis, and filling of the application to getting the MD 5 License to Manufacture Medical Devices and IVDs.
How to know your Device Classification?
The manufacturer should classify the devices as defined in Chapter 2 of the Medical Device Rules, 2017 based on the risk involved in the device. Class A devices are simple and involve the lowest risk and with minimum potential of harm. Class B devices are with low moderate risk and with a high potential of harm compared to Class A.
CDSCO publishes the list of notified devices with their classification on its website. You can download the list of notified devices from our download page.
Form MD 3 is the application form to be submitted by the manufacturer of Class A or Class B Devices to the State Licensing Authority for the grant of MD 5 License to Manufacture Medical Devices and IVDs in India.
Form MD 5 is the license granted to the manufacturer of Class A or Class B Devices by the State Licensing Authority to manufacture and sale of medical devices including in-vitro diagnostic devices in India.
Companies that intend to manufacture Class A or Class B medical devices and in-vitro diagnostic devices on their premises can apply for MD 5 License.
To legally manufacture Class A or Class B medical devices or in-vitro diagnostic devices in India, you need an MD 5 License.
In accordance with Medical Device Rules, 2017 and CDSCO, the applicant must apply for the MD-5 License with the following mandatory documents:
- Application Form (MD-3)
- Site master file
- Device master file
- ISO 13485 Certificate
- Receipt of the Fee
- Company Identity
- Partnership deed / Memorandum of Association
- List of directors/partners
- Sale deed/rent deed
- Performance evaluation report (if IVDs)
- Test License, if any
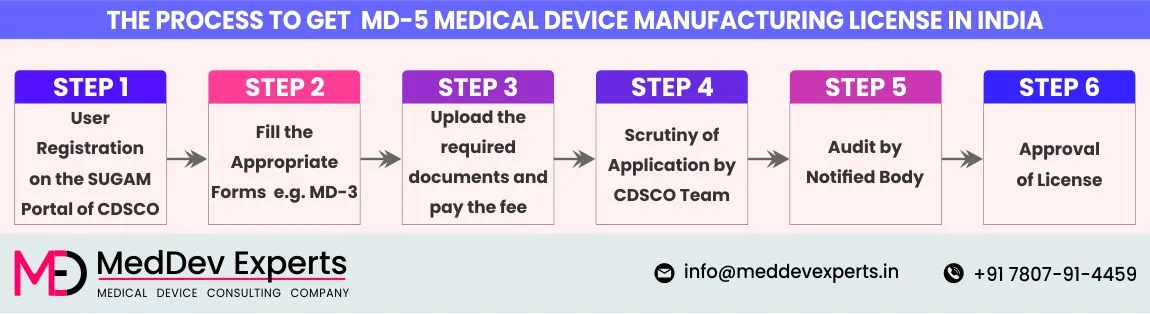
- Step 1 User registration on SUGAM Portal of CDSCO: Register your organization on CDSCO’s SUGAM Portal.
- Step 2 Fill out the Application Form MD-3: Fill in the respective application form (MD-3) and submit it through CDSCO’s online SUGAM Portal.
- Step 3 Upload the Required Documents & Pay the Fee: Upload the necessary documents like the site master file, device master file, and other documents, and make the required payment.
- Step 4 Scrutiny of Application by the Licensing Authority: Your application will be scrutinized by the State Licensing Authority. It will proceed to a quality management system compliance audit if it meets the requirements.
- Step 5 Audit by Notified Body: Your premises will be audited by an independent notified body approved by the CDSCO. If any Non-Conformance (NC) is identified, you will be required to submit an NC closure report.
- Step 6 Approval of MD-5 License: Once all the conditions are met, the state licensing authority will grant the license.

We have prepared a detailed process on how to get Medical Device Manufacturing License in India. The blog covers all the details of the application process and a step-by-step guide to applying for the license. If you need any assistance on the application process, please feel free to contact us. We will be happy to help you.
Conditions for MD 5 License
You must:
- Show your license to the Medical Device Officer or other senior officer when requested.
- Report any serious adverse events and actions taken to the State or Central Licensing Authority within 15 days.
- Get prior approval from the State Licensing Authority for any major changes to your manufacturing process.
- Inform the State or Central Licensing Authority of any minor changes to your manufacturing process within 30 days.
- Test each batch of products before releasing them for sale.
- Withdraw any batch of products found to be non-conforming with the Act and rules, and recall any affected products already sold, if instructed to do so by the State Licensing Authority.
- Keep an audit or inspection book for the Notified Body or Medical Device Officer to record their observations and any non-conformities.
- Keep at least one unit of sample from each batch of invasive medical devices and in vitro diagnostic medical devices manufactured for reference purposes for 180 days after the batch expiry date.
- Keep records of manufacturing and sales that are open to inspection by a Medical Device Officer.
- Include a package insert or user manual with medical devices when offered for sale, if applicable.
- Only manufacture or test medical devices under the direction and supervision of competent technical staff.
- Inform the State Licensing Authority if you stop manufacturing activity or close your manufacturing site for 30 days or more.
By following these conditions, you can ensure that your MD5 license is valid and that you are operating in compliance with the law.
Change in constitution
If you have a license to manufacture medical devices, and your company changes ownership or structure, you must tell the State Licensing Authority within 45 days. You must also apply for a new license within 180 days. Your old license will still be valid until you get a new one, or your application is rejected. If your application is rejected, you can appeal to the State Government within 60 days.
State Licensing Authority Conducts Unannounced Inspections of Medical Device Manufacturing Sites
The State Licensing Authority may inspect medical device manufacturing sites without warning. These inspections will be done by a Medical Device Officer, and will happen at least 2% of the time.
Suspension and Cancellation of Medical Device License: What You Need to Know
If a medical device manufacturer breaks the rules, the government can suspend or cancel their license. The manufacturer will have a chance to explain why their license should not be suspended or cancelled before a decision is made.
If a manufacturer’s license is suspended or cancelled, they can appeal to the government within 45 days. The government will give the manufacturer a hearing before making a decision. The government can revoke a suspension order if they have a good reason to do so.
To get MD 5 Medical Device Manufacturing License, the Quality Management System of the manufacturing site must be certified with ISO 13485 or ICMED 13485. The manufacturer must appoint competent technical persons both for the manufacturing and testing of the devices.
The fees for MD 5 License
The fee is covered in the Second Schedule of the Medical Device Rule, 2017. For MD 5 License to Manufacture Class A or B Medical Devices and IVDs in India, the manufacturer is to pay ₹ 5,000/- per site and ₹ 500/- for each distinct device.
Validity & Renewal of MD 5 License
The license once issued will remain valid indefinitely, as long as the applicant pays the retention fee every five years from the date of issue of the license.
Timeline to get MD-5 License
It can take 3 to 6 months to get MD-5 License from the state licensing authority.
Who is the consultant for MD-5 License?
MedDev Experts, a regulatory consultant with 15+ years of experience in helping companies get MD-5 Licenses.
Preparing for Notified Body Audit for MD 5 License
Your manufacturing facility is slated for an audit by a CDSCO Registered Notified Body to verify adherence to the Medical Device Rules, 2017. This comprehensive audit spans crucial aspects such as facility readiness, quality management systems, device master files, risk management, validation, testing, and manufacturing processes.
Attaining your MD 5 License and tapping into the burgeoning Indian medical device market is an achievable goal. Although navigating the Notified Body audit may seem daunting, fear not – we are here to provide you with the tools and knowledge necessary to conquer the summit!
Here are 5 powerful steps to ensure a smooth and successful Notified Body audit:
1. Paperwork Perfection: Polish Your Paperwork for a Polished Audit
- Thoroughly examine your quality management systems, quality manual, manufacturing processes, product specifications, risk management plan, device master file, and site master file.
- Verify that your documentation is current, precise, and in compliance with the latest MD 5 License requirements. Avoid stumbling over inconsistencies or outdated information.
- Enhance your QMS to meet international standards such as ISO 13485 or ICMED 13485. Impress auditors with strong design control, risk management, and post-market surveillance processes.
2. Prepare Your Facility: Convert Your Space to Meet GMP Standards
- Revamp your manufacturing facility to adhere to Good Manufacturing Practices (GMP), prioritizing cleanliness, organization, and functionality.
- Promptly resolve any non-compliance issues.
- Keep in mind that Notified Body auditors meticulously examine your environment, so ensure it is in top-notch condition!
3. Device Testing: Demonstrate the Excellence of Your Products
- Subject your medical devices to rigorous testing and validation, ensuring compliance with specified standards, including biocompatibility, sterilization, and other pertinent aspects.
- Eliminate any doubt by conducting thorough testing, showcasing your unwavering commitment to quality and safety.
4. Risk Mitigation Mastery: Excelling in the Art of Averting Mishaps
- Master the art of risk management! Identify, assess, and proactively mitigate potential risks associated with your devices.
- Clearly document your risk assessments and mitigation strategies to underscore your dedication to patient safety.
5. Team Empowerment: Cultivate a Squad of Quality Champions
- Invest in your team’s expertise! Provide training on the latest regulatory requirements and ensure competence in implementing quality procedures.
- An adept and knowledgeable team not only impresses auditors but also guarantees consistent quality.
By adhering to these 5 impactful steps, you can turn your Notified Body audit from a daunting challenge into a triumphant opportunity.
MedDev Experts guides you through every step of the audit process, ensuring compliance with Medical Device Rules, 2017. Our experts assess facility readiness, quality management systems, device master files, risk management, validation, testing, and manufacturing processes.
Schedule a free consultation today and learn how we can help you achieve smooth MD 5 License approval.
ISO 13485:2016 Medical Device Quality Management System is a mandatory equipment for all manufacturers of medical devices and in-vitro diagnostic devices. ISO 13485:2016 outlines the prerequisites for establishing a quality management system.
This system is essential for organizations looking to demonstrate their competence in consistently delivering medical devices and associated services that not only align with customer expectations but also comply with relevant regulatory mandates.
These organizations may be engaged in various stages of a product’s lifecycle, encompassing design and development, manufacturing, warehousing, distribution, installation, servicing of medical devices, as well as the design, development, or provision of complementary functions (e.g., technical support).
Furthermore, ISO 13485:2016 can serve as a valuable reference for suppliers and external entities that provide products or quality management system-related services to such organizations.
MedDev Experts provides ISO 13485 Certification Services. Contact us now to get certified.
How MedDev Experts can help you get an MD 5 License
MedDev Experts is a leading medical device consulting company in India that provides services to help companies obtain MD 5 Licenses. We offer a comprehensive range of services, including:
- Application Form MD-3 Submission: We will help you complete and submit Form MD-3, the application form for the Grant of Manufacturing License for Class A and B medical devices and in-vitro diagnostic devices.
- SOP and Format Development: We will guide you in developing comprehensive Standard Operating Procedures (SOPs) and documentation formats that align with your Quality Management System (QMS) requirements and regulatory standards.
- QMS Certification: We will help you create and establish a robust QMS tailored to meet ISO 13485 standards and provide ISO 13485 certification.
- Mock Audit Preparation: We will conduct a mock audit internally to identify areas for improvement and ensure readiness for the upcoming audit by Notified Bodies.
- Audit by Notified Bodies: We will facilitate and actively participate during the audit process conducted by the Notified Body, ensuring all compliance measures are met.
- SUGAM Portal Query Responses: We will guide you in preparing and submitting detailed responses to any queries raised by the licensing authority during the licensing process.
Are You Looking for MD-5 License to Manufacture Medical Devices & IVDs in India?
Contact MedDev Experts today! We have a team of experienced professionals who can help you navigate the complex regulatory process and get your MD-5 license approved.
Why choose MedDev Experts?
- We have over 15 years of experience in medical device regulatory compliance services.
- We have a team of experienced and qualified professionals who are experts in medical device regulatory compliance.
- We offer a comprehensive range of services that covers all aspects of the MD 5 licensing process.
- We are committed to providing our clients with high-quality services and support.
Let’s Get Started on Your Medical Device Licensing Journey