The MD 9 Medical Device License is a mandatory requirement for manufacturers of Class C and Class D medical devices in India. It is issued by the Central Drugs Standard Control Organisation (CDSCO).
To obtain an MD 9 License, manufacturers must meet certain requirements, including having a qualified and experienced staff, a suitable manufacturing facility, and a quality management system. The application process for an MD 9 License is complex and can take several months to complete.
If you are a manufacturer of Class C or Class D medical devices in India, you must obtain an MD 9 License in order to legally operate your business. To learn more about the MD 9 Medical Device License and the application process, contact MedDev Experts now.
MedDev Experts provides services to apply and get MD 9 License from the CDSCO to manufacture Class C and D Medical Devices including In-Vitro Diagnostic Devices. We provide a one-stop solution from start to grant of MD 9 license.
Attention Medical Device Manufacturers in India!
Last Chance to Apply for MD 9 Medical Device License for Class C and D Devices
The Ministry of Health and Family Welfare has granted a transition period of 42 months for obtaining an MD 9 Medical Device License for Class C and Class D Devices. This means that you have until September 30, 2023 to apply for the license. After this deadline, it will be mandatory for all manufacturers to have an MD-9 License in order to manufacture, sell, or distribute Class C and Class D medical devices in India.
The transition period, specified in Notification No. GSR 102 (E), is set to conclude on September 30, 2023.
Don’t miss out on this opportunity! Apply for your MD 9 Medical Device License today.
How Medical Devices are Classified in India
Medical devices in India are classified into four classes based on the level of risk they pose to patients. This classification system is defined in the Chapter 2 of the Medical Device Rules, 2017. All medical devices, including in-vitro diagnostic devices, are subject to this classification system.
Class A
- Low risk
- Low complexity
- Minimal potential harm
- Examples: tongue depressors, bandages, and surgical masks
Class B
- Low to moderate risk
- Moderate complexity
- Greater potential for harm if not used properly
- Examples: syringes, dental cement, pregnancy detection kits, and nebulizers
Class C
- Moderate to high risk
- High complexity
- Higher potential for harm if not used correctly or if any malfunctions occur
- Examples: implantable devices, surgical lasers, and cardiac pacemakers
Class D
- High risk
- Highly complex and invasive
- Significant potential for harm if not used appropriately
- Examples: heart valves, advanced surgical instruments, life-supporting devices
The Central Licensing Authority (CLA) classifies medical devices based on their intended use and specified parameters, and publishes the List of Notified Medical Devices on the Central Drugs Standard Control Organisation (CDSCO) website. You can download the list from our Download Section.
What is Form MD 7?
- MD 7 is the legal application form to apply for a license to manufacture Class C or Class D medical devices in India.
- It is issued by the Central Drugs Standard Control Organisation (CDSCO).
- The manufacturers must apply with MD 7 to get MD 9 License to manufacture Class C or Class D medical devices in India.
What is Form MD 9?
- MD 9 is the legal license granted by CDSCO to manufacture Class C or Class D medical devices in India.
- It is issued after the CDSCO has reviewed and approved the application form.
- Manufacturers of Class C or Class D medical devices in India must have an MD 9 medical device license in order to legally manufacture, sell, or distribute their products.
Requirements for Obtaining MD 9 Medical Device License for Class C and Class D Devices
In order to obtain an MD 9 Medical Device License for Class C and Class D Devices, the applicant must meet the following requirements:
- The manufacturing site must comply with the Quality Management System ISO 13485 or ICMED 13485.
- Compliance with environmental requirement.
- All mandatory documents for MD 9 License.
- The applicant must appoint competent technical staff for the manufacturing and testing of medical devices.
Manufacturing Staff
The manufacturing staff must have the following qualifications and experience:
- A degree in engineering, pharmacy, or science from a recognized university and at least 2 years of experience in manufacturing medical devices.
- A diploma in engineering or pharmacy from a recognized institute and at least 4 years of experience in manufacturing medical devices.
Testing Staff
The testing staff must have a degree or diploma in engineering, pharmacy, or science and at least 2 years of experience in testing medical devices.


Documents to be submitted to CDSCO for MD 9 Medical Device License
- An application Form MD 7
- Covering Letter
- Constitution of the Firm
- Sale Deed / Rent Deed
- ISO 13485 Certificate
- Quality Manual
- Quality Management System Procedures
- Site Master File
- Executive Summary of the Device
- Device Description and product specifications, including variants and accessories
- Product Specification
- Reference to predicate or previous generations of the device
- Labels and Instruction for Use
- Device Design and Manufacturing Information
- Essential Principles Checklist
- Risk analysis and control summary
- Verification and validation of the medical device
- Design verification and validation
- Biocompatibility validation data
- Medicinal substances data (if device contains Drug)
- Biological Safety
- Sterilization Validation data
- Software verification and validation (if software used)
- Animal studies – Preclinical data
- Stability validation data
- Clinical evidence
- Post Marketing Surveillance data
- Certificate of Analysis of finished product for minimum 3 consecutive batches
- Prescribed Fee
How to Apply for a Medical Device MD 9 License
While making an application to obtain MD 9 Medical Device License for Class C and Class D Devices, the applicant must fulfil the following requirements:
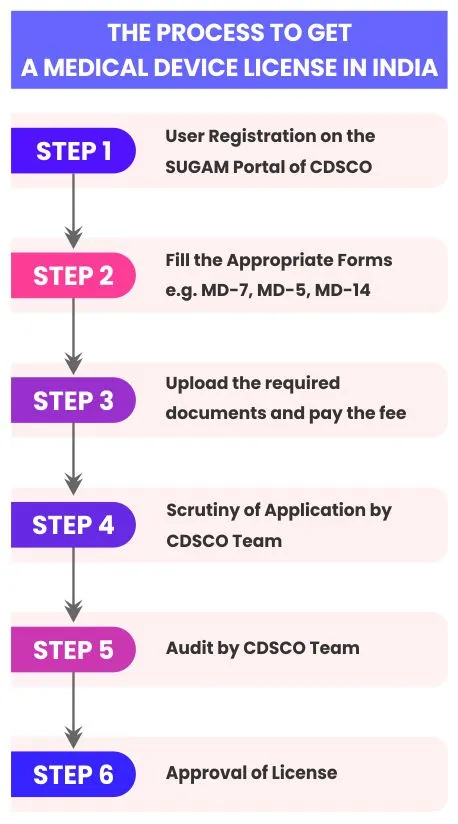
Step 1: Register your organization on the SUGAM Portal of CDSCO.
Step 2: Apply for the MD-9 Medical Device License for Class C and Class D Devices. The application should be submitted on Form MD-7 through the online SUGAM Portal of CDSCO.
Step 3: Upload the required documents and pay the fee. The required documents include Memorandum of Association, Sale/Rent Deed, Plant Layout, Site Master File, Device Master File for each product, Performance evaluation report, etc.
Step 4: Your application will be scrutinized by the CDSCO Team for compliance. If your application is found in order, it will be processed for quality management system compliance audit by the CDSCO Team.
Step 5: The CDSCO Team will conduct the audit at the applicant’s manufacturing premises. If any Non-Conformance (NC) is raised by the audit team, it will be notified through the SUGAM Portal of CDSCO and you have to submit NC closure report along with evidence.
Step 6: Once all the conditions of the MD-9 Medical Device License for Class C and Class D Devices are complied with, the license is to be granted by the CDSCO.
Are You Looking for MD 9 License to Manufacture Class C or D Medical Devices & IVDs in India?
Contact MedDev Experts today! We have a team of experienced professionals who can help you navigate the complex regulatory process and get your MD 9 license approved.
The cost of an MD 9 Medical Device License in India
The manufacturing license to manufacture Class C or D medical devices in India costs ₹ 50,000. An additional ₹ 1,000 will be charged for each distinct medical device.
How long does it take to get the MD 9 License?
It can take 3 to 6 months to get an MD 9 License to manufacture Class C or D Devices from the CDSCO.
MD 9 Medical Device License Validity
A license issued in Form MD-9 shall remain valid indefinitely, as long as the applicant pays the retention fee every five years from the date of issue of the license.
MD 9 Medical Device License Renewal
To keep your MD 9 Medical Device License valid, you must pay the retention fee every five years from the date of issue.
How does MedDev Experts assist in obtaining MD 9 Medical Device License for Class C-D Devices in India?
- We will check and guide you to classify your devices depending on their risk and give you clear guidance.
- We will conduct a pre-audit to check whether or not your manufacturing unit complies with CDSCO requirements.
- We will help and assist you to prepare your Device Master File as per Medical Device Rule.
- We will guide you to prepare and comply with the Audits by CDSCO.
- If any query is raised by the assessing team of regulatory body, we will guide you to submit compliance for the raised query.
- We provide reliable and on-time complete end-to-end guidance to get your Medical Device Licence at an affordable cost.
How to comply with the Quality Management System for MD 9 License?
To comply with the Quality Management System for MD 9 License, you can follow these steps:
- Get ISO 13485:2016 certified. ISO 13485 is an international standard that sets out the requirements for a quality management system for the design, development, production, installation, and servicing of medical devices. Getting ISO 13485 certified is the best way to demonstrate to the CDSCO that your quality management system meets the requirements of the MD 9 License.
- Implement a quality management system that complies with the requirements of ISO 13485:2016. This includes developing and implementing procedures for all aspects of your medical device business, such as product design, development, manufacturing, quality control, and customer service.
- Maintain your quality management system. This includes conducting regular internal audits to ensure that your system is still effective and making any necessary changes to improve your system.
You can also contact MedDev Experts to help you comply with the Quality Management System for MD 9 License. We can help you develop and implement a quality management system, get ISO 13485 certified, and maintain your quality management system.
Additional tips for complying with the QMS for MD 9 License:
- Make sure that your quality management system is documented and that all employees are trained on the procedures.
- Conduct regular risk assessments to identify and mitigate potential risks to the quality of your medical devices.
- Get regular external audits from a qualified auditor to ensure that your quality management system meets the requirements of ISO 13485:2016.
By following these tips, you can ensure that your quality management system meets the requirements of the MD 9 License and that you are producing high-quality medical devices.
Passing Your CDSCO Audit for MD 9 License: Key Steps for Manufacturers
The CDSCO conducts a thorough audit of your manufacturing premises to ensure compliance with Medical Device Rules, 2017. This audit covers critical areas like facility readiness, quality management systems, device master files, risk management, validation, testing, and manufacturing processes.
Obtaining your MD 9 License and entering the thriving Indian medical device market is within reach. But navigating the CDSCO audit can feel like scaling Mount Everest. Don’t worry, we’re here to equip you with the tools and knowledge to reach the summit!
Here are 5 powerful steps to ensure a smooth and successful CDSCO audit:
1. Documentation Detox: Polish Your Paperwork for Perfection
- Conduct a comprehensive review of your quality management systems, quality manual, manufacturing processes, product specifications, risk management plan, device master file, and site master file.
- Ensure your documentation is up-to-date, accurate, and aligned with the latest MD 9 License requirements. Don’t let inconsistencies or outdated info trip you up!
- Upgrade your QMS to international standards like ISO 13485. Robust design control, risk management, and post-market surveillance processes impress auditors.
2. Facility Readiness: Transform Your Space for GMP Compliance
- Give your manufacturing facility a thorough makeover to comply with Good Manufacturing Practices (GMP). Cleanliness, organization, and functionality are key.
- Address any non-compliance issues promptly.
- Remember, CDSCO auditors scrutinize your environment, so make it shine!
3. Testing of Devices: Prove Your Products' Prowess
- Put your medical devices through their paces with rigorous testing and validation. Ensure they meet all specified standards and have undergone testing for biocompatibility, sterilization, and other relevant aspects.
- Leave no room for doubt – thorough testing demonstrates your commitment to quality and safety.
- Become a risk management ninja! Identify, assess, and proactively mitigate potential risks associated with your devices.
- Document your risk assessments and mitigation strategies clearly to showcase your dedication to patient safety.
5. Training Needs: Build a Team of Quality Crusaders
- Invest in your team’s expertise! Train your staff on the latest regulatory requirements and ensure they’re competent in implementing quality procedures.
- A well-trained and knowledgeable team impresses auditors and guarantees consistent quality.
By following these 5 powerful steps, you’ll transform your CDSCO audit from a daunting challenge into a triumphant opportunity. Remember, with meticulous preparation and expert guidance, securing your MD 9 License and launching your medical devices in India is a dream you can hold in your hands.
Concerned about the upcoming CDSCO Audit?
MedDev Experts guides you through every step of the audit process, ensuring compliance with Medical Device Rules, 2017. Our experts assess facility readiness, quality management systems, device master files, risk management, validation, testing, and manufacturing processes.
Schedule a free consultation today and learn how we can help you achieve smooth CDSCO approval.
How to Renew Your MD 9 License: Process & Required Documents
Renewing your MD 9 License is crucial to maintain compliance and continue manufacturing or selling medical devices in India. Here’s a breakdown of the key deadlines and fees to avoid penalties or license cancellation:
- 5-Year Renewal Period: Your MD 9 License must be renewed every 5 years from the original date of issue.
- Grace Period for Late Payments: If you miss the renewal deadline, you have a 180-day grace period to pay the renewal fee, but a late fee of 2% per month will be added.
- License Cancellation: Failure to pay the renewal fee within 180 days will result in automatic cancellation of your license.
Renew Your MD 9 License Effortlessly: A Step-by-Step Guide for Manufacturers
Want to ensure uninterrupted manufacturing of your medical devices in India? Renewing your MD 9 License is key! Here’s the simple way to do it online:
- Head to the SUGAM Portal: This is the official platform for medical device licensing in India.
- Choose “Retention”: Let the system know you’re not applying for a new license, but renewing your existing one.
- Download the Guide: For step-by-step instructions with screenshots, grab the handy guide right on the SUGAM Portal.
That’s it! Just follow the clear instructions and submit your application along with the required documents. It’s smooth sailing from there!
Remember: Staying compliant with MD 9 license renewal keeps your business running smoothly and avoids any hassles. So, start the process today and enjoy peace of mind!
Documents required to get renew of MD 9 License
To renew the MD 9 License, the applicant should provide the following documents:
Just write a quick letter to the licensing authority explaining you’re requesting retention of your MD 9 License. Be sure to list all the devices covered and mention the fee payment details.
To renew your MD 9 License, just submit a signed statement saying your company structure (Constitution of the Firm) and product information (Plant Master File & Device Master File) haven’t changed.
To renew your MD 9 License, showcase your expert technical staff who ensures top-notch device manufacturing. Just share details of your technical staff like:
- Engineers or scientists: With a relevant degree and at least 2 years of experience in medical device manufacturing or testing.
- Pharmacists or engineers with diplomas: In relevant fields, and at least 4 years of experience in making or testing medical devices.
Don’t forget! Include details of someone with a degree/diploma and at least 2 years of testing experience to oversee those important quality checks.
To keep your MD 9 License going strong, tell the story of your commitment to safe devices. Just include the following in your renewal:
- Sales Data: Share the past 5 years of your medical device sales – transparency builds trust!
- Customer Complaint: List any complaints received in the last 5 years – showing you address customer complaint is key.
- Responsible Recalls: If you ever had to recall a product, briefly explain why and what you did to improve. It demonstrates proactive care.
- Continuous Improvement: Did you implement any corrective and preventive actions (CAPA) for any concerns? Share them – it showcases your dedication to quality.
You need to submit the copy of existing manufacturing license for which retention is applied.
Did you modify your medical device after getting your MD 9 License? No worries! Just include details of any “post-approval changes” you made, like new features or adjustments. It helps keep everything up-to-date and your license secure.
Frequently Asked Questions for MD 9 Medical Device License
Who can apply for MD 9 License?
Companies that intend to manufacture Class C or Class D medical devices and in-vitro diagnostic devices on their premises can apply for MD 9 License.
Why do you need an MD 9 Medical Device License?
To legally manufacture Class C or Class D medical devices or in-vitro diagnostic devices in India, you need an MD 9 License.
Where can I get the Device Master File (DMF) for the MD 9 License?
MedDev Experts provides the Device Master File for medical devices and in-vitro diagnostic medical devices.
Where can I get the Plant Master File required by the CDSCO?
MedDev Experts provides customized and CDSCO-compliant plant master files.
How long does it take to get the MD 9 License?
It can take 3 to 6 months to get an MD 9 License to manufacture Class C or D Devices from the CDSCO.
Who issues the MD 9 license?
The MD 9 license is issued by the Central Drugs Standard Control Organisation (CDSCO) in India to manufacture Class C or D medical devices and in-vitro medical devices.
What is the difference between Form MD 7 and MD 9?
Form MD 7 is the application form to get a license to manufacture Class C or Class D devices and Form MD 9 is a legal license issued by the CDSCO to the applicant to manufacture Class C or D devices in India.
Can I change the manufacturing location after obtaining an MD 9 License?
Any change in the manufacturing location can be done through post-approval changes.
Our Services
We offer a comprehensive service of medical device licensing and registration in India, including:
- Medical Device Registration and License
- Medical Device Import License
- Medical Device Wholesale License
- ISO Certification
- GMP Compliance
- FDA Product Listing
- 510(k) Submission
- CE Documentation
- BIS Certification (ISI Marking)
- Import License for Cosmetic Products
- Medical Device Labeling Compliance
- Cosmetic Import License from CDSCO
Who can resolve the queries raised by the CDSCO for the MD 9 License?
MedDev Experts can resolve the queries raised by the CDSCO for the MD 9 License.
We are a specialized medical device consulting company with more than 15 years of experience. We can help you understand the requirements of the MD 9 License application process and guide you through the process of resolving any queries raised by the CDSCO.
If you are having trouble resolving a query raised by the CDSCO, I recommend that you contact MedDev Experts for assistance. They have a team of experienced consultants who can help you to get your MD 9 License at the earliest possible time.
Who is the consultant for MD 9 License?
MedDev Experts is a consultant for the MD 9 License. We are a regulatory consultant with more than 15 years of experience in providing services to help companies obtain MD 9 Licenses.
MedDev Experts can help you with all aspects of the MD 9 License application process, including:
- Preparing the application documentation
- Responding to queries from the CDSCO
- Resolving any non-compliance issues
- Ensuring that your application meets all of the requirements
If you are applying for an MD 9 License, we recommend to contact us for a free assistance. We can help you to get your license at the earliest possible time and ensure that your application is successful.
How MedDev Experts can help you get an MD 9 License
MedDev Experts is a leading medical device consulting company in India that provides services to help companies obtain MD 9 Licenses. We offer a comprehensive range of services, including:
- Application Form MD 7 Submission: We will help you complete and submit Form MD 7, the application form for the grant of license to manufacture Class C or D medical devices and in-vitro diagnostic devices.
- SOP and Format Development: We will guide you in developing comprehensive Standard Operating Procedures (SOPs) and documentation formats that align with your Quality Management System (QMS) requirements and regulatory standards.
- QMS Certification: We will help you create and establish a robust QMS tailored to meet ISO 13485 standards and provide ISO 13485 certification.
- Mock Audit Preparation: We will conduct a mock audit internally to identify areas for improvement and ensure readiness for the upcoming audit by Notified Bodies.
- Audit by CDSCO: We will facilitate and actively participate during the audit process conducted by the CDSCO, ensuring all compliance measures are met.
- SUGAM Portal Query Responses: We will guide you in preparing and submitting detailed responses to any queries raised by the licensing authority (CDSCO) during the licensing process.
Why choose MedDev Experts?
- We have over 15 years of experience in medical device regulatory compliance services.
- We have a team of experienced and qualified professionals who are experts in medical device regulatory compliance.
- We offer a comprehensive range of services that covers all aspects of the MD 9 licensing process.
- We are committed to providing our clients with high-quality services and support.
Book a
FREE CONSULTATION
If you are looking for MD 9 License to manufacture Class C or D devices and in-vitro diagnostic devices, please contact us today. We would be happy to help you through the process. Our dedicated team is here to answer your questions, provide guidance, and support you throughout the registration process.
Phone
+91-78079-14459








