How to get Medical Device Manufacturing License in India in 2023
Medical Device Manufacturing License in India
Medical Device Rule, 2017 came into force to govern the import, manufacture, sale and distribution of Medical Devices including In-Vitro Diagnostic Devices in India. The Medical Device Rule is introduced to ensure medical devices’ safety, effectiveness, and quality and to align India’s regulatory framework with international standards. The rules came into force with effect from 1st January 2018.
Table of Contents
ToggleLicensing Authority
In simpler terms, there are two licensing authorities responsible for issuing licenses to manufacture medical devices and IVDs in India.
The Central Licensing Authority
The Central Licensing Authority (CDSCO) is responsible for issuing medical device import license, MD 9 License to manufacture Class C and Class D medical devices and IVDs, medical device test license, and the approval of new medical devices and in vitro diagnostic medical devices.
The State Licensing Authority
The State Licensing Authority (State FDA) is responsible for issue of MD 9 License to manufacture Class A or Class B Medical Devices including in vitro diagnostic medical devices, as well as the MD 42 License to sale, stock, exhibit or offer for sale or distribution of medical devices of all classes.
Classification of Medical Devices
Medical devices including in-vitro diagnostic medical devices are categorized into four classes based on their intended use and the level of risk they pose to the patient.
- Class A: Low Risk
- Class B: Low Moderate Risk
- Class C: Moderate High Risk
- Class D: High Risk
Application for manufacture for sale or for distribution of Class A or Class B medical device
If you want to get the MD 5 Medical Device Manufacturing License to manufacture Class A or Class B medical devices, you must submit your application to the State Licensing Authority on CDSCO SUGAM online portal. The applicant needs to fill out Form MD-3 for the manufacturing license on their own premises or Form MD-4 for the manufacturing license on loan premises. The MD 5 License and MD 6 Loan Licenses are issued by the State Licensing Authority to manufacture Class A or Class B Devices in India.
Application for manufacture for sale or for distribution of Class C or Class D medical device
If you want MD 9 Medical Device Manufacturing License to manufacture a Class C or Class D medical device, you must submit your application to the Central Licensing Authority. The applicant needs to fill out Form MD-7 for the manufacturing license on their own premises or Form MD-8 for the manufacturing license on loan premises. MD 9 License and MD 10 Loan License will be issued by the CDSCO after verification of documents and audit compliance.
User Registration
To apply for the Medical Device Manufacturing License (MD 5 License and MD 9 License), you need to register with the https://cdscomdonline.gov.in/ portal. Following documents are required to register your organization on the portal.
- ID Proof (Aadhar Card/PAN Card/Passport)
- Undertaking
- Address Proof (Certificate of incorporation/UDYAM)
- Copy of the Manufacturing or wholesale license, if any.
Once your registration is complete, you can apply for the Medical Device Manufacturing License.
Application Process for Medical Device Manufacturing License
Open the Chrome Browser, search for cdscomdonline, click on the first search result to go to the portal. Or you may type the website url to go to the portal “https://cdscomdonline.gov.in” for application of Medical Device Manufacturing License
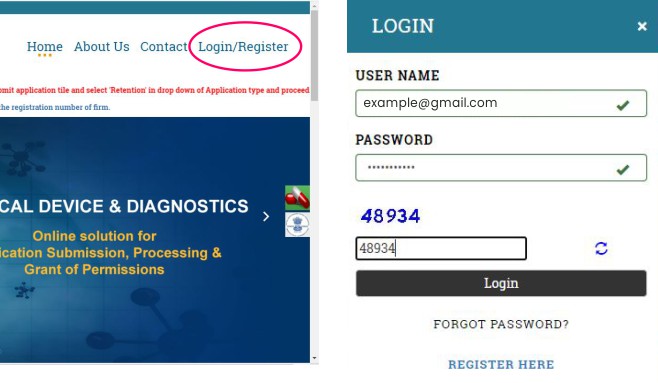
Click on the Login/Register link on the top-right corner of the portal. A new pop-up login window will appear. Fill in your username, password, and enter the captcha. Then click on the LOGIN Button.
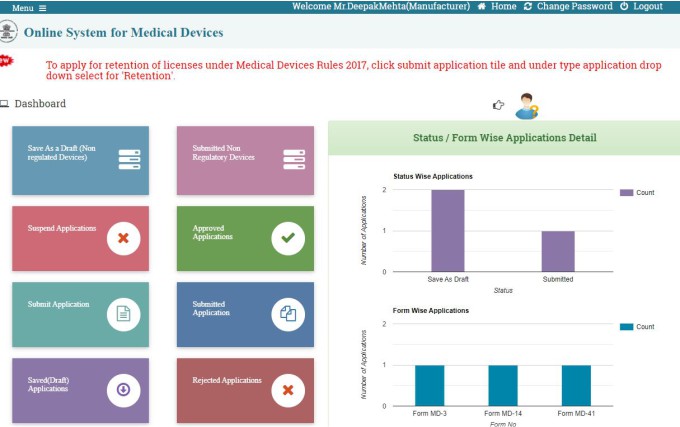
Then you will be redirected to the Online System for Medical Device dashboard for Medical Device Manufacturing License. Click on SUBMIT APPLICATION Section.
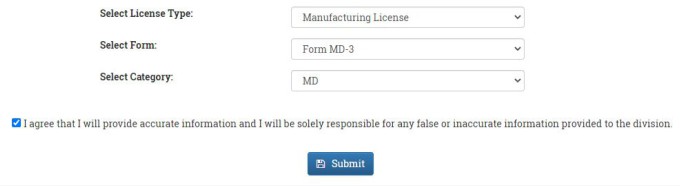
Select the License type from the drop-down menu. In our case select Manufacturing License. Select the Form from the drop-down menu. In our case select Form MD-3. Select the Catagory from the drop-down menu. In our case select MD. Then accept the terms and conditions of the portal and then press the SUBMIT button.
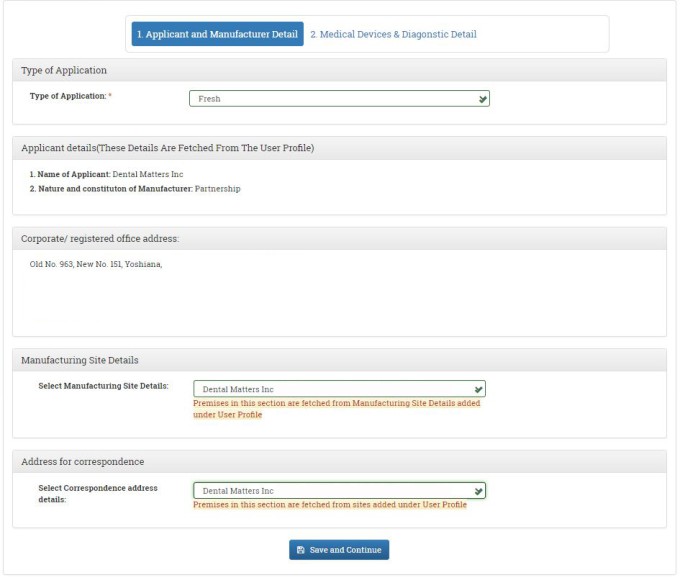
In the Next window, you have to fill in the applicant and manufacturer details. Select the type of Application from the drop-down menu. In our case select FRESH. The applicant details and your address will be fetched from the user profile. Select the manufacturing site details from the drop-down menu. Select correspondence address from the dropdown menu. After filling in all the details, click on SAVE AND CONTINUE Button.
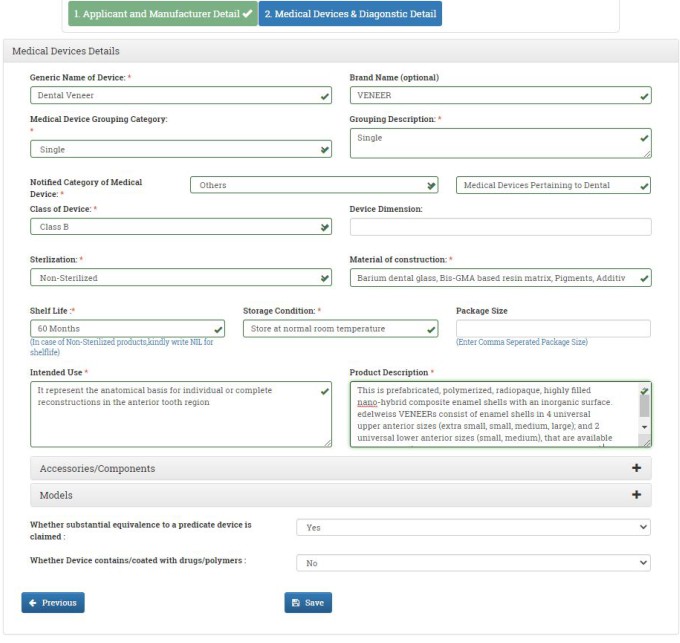
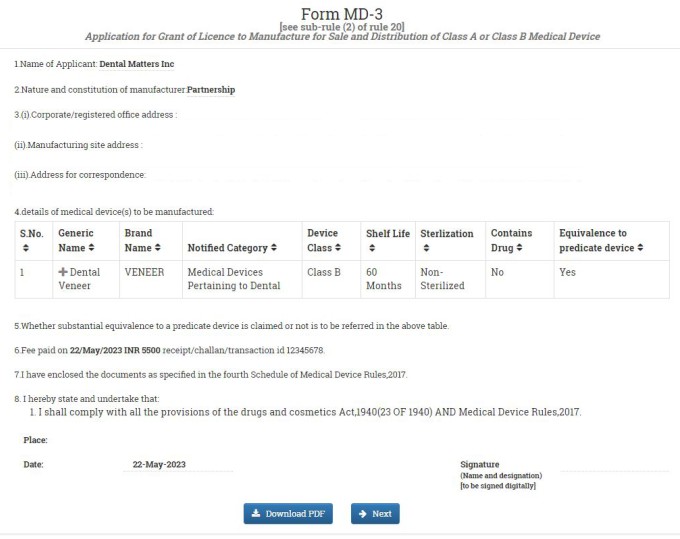
In the next window, you have to fill in the medical device and diagnostic detail. Fill in the Generic Name and Brand Name of the Device. Select the Medical Device Grouping Category from the drop-down menu. In our case, we are selecting Single. Mention the grouping description.
Select the Notified Category of Medical Device. If your notified category is not listed, select other and type manually. In our case, we are selecting others and our notified category is Medical Devices Pertaining to Dental.
Select the classification of the device from the drop-down menu. Confirm if the device is sterile, non-sterile or both from the dropdown menu. Mention the material of construction of the device. Write the shelf life of the device. Mention storage condition of the device.
Write intended use of the device. Enter device description. Confirm whether substantial equivalence to a predicate device is claimed for your device. Confirm whether the device contains drugs. After filling all the details, click on SAVE button. You can add as many devices as you want. Once you added all your devices, click on NEXT button.
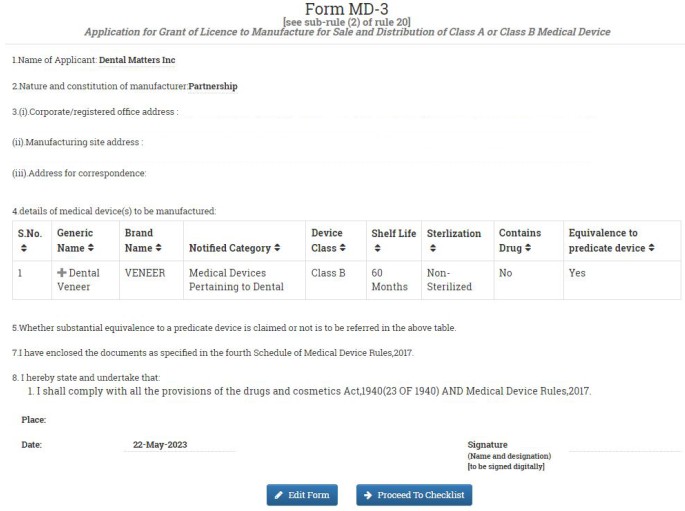
In the next window, you will get a preview of you Form MD-3 Application. If you want to make any correction, click on EDIT FORM or click on PROCEED TO CHECKLIST.
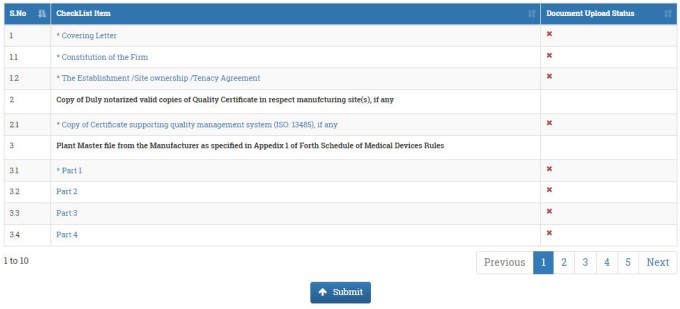
In the next window, you have to upload all the necessary documents. The documents which are marked with *, it means it is compulsory to upload. Upload only pdf format. When you uploaded the documents, the document upload status will be changed to green tick.
First select covering letter, a new window will be opened. Chose the file which you want to uploaed. Fill the remarks. In the remarks section you may write the document title, covering letter. Then click on SUBMIT button. Similarly, upload all the required documents. Once you uploaded all the documents, click on SUBMIT button.
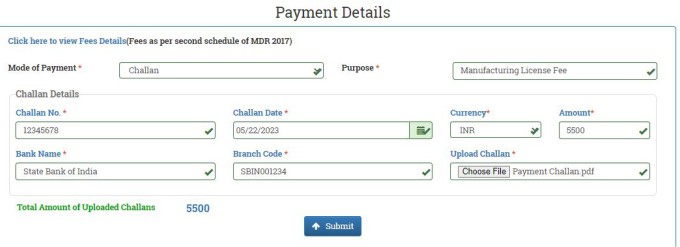
In the next window, you will be asked to fill the payment details. Select mode of payment to Challan. Fill the Purpose as Manufacturing License Fee. Enter Challan Number and Challan Date. Select the Currency from the dropdown menu to INR. Fill the Amount you have paid. Enter Bank Name and Brach Code. Upload a copy of the payment challan.
For Class A & B devices, the payment to be made to the State Licensing Authority. For Class B and C devices, the payment will be paid to the Central Licensing Authority.
After filling all the payment details, click on SUBMIT button. The legal form will be displayed in the next window. Click on download PDF button. The legal form will be downloaded. Once the download process is completed, click on NEXT Button.
Digitally signature the Legal Form and chose the file to upload. Then click on SUBMIT button. On submission, you will get your application number for further communication with the Licensing Authority.
We hope this blog has provided you with a comprehensive overview of medical device manufacturing licenses in India. Whether you are a domestic manufacturer or an international player looking to enter the Indian market, working closely with regulatory bodies and seeking expert advice will help you navigate the licensing process effectively.
If you require any further assistance or have inquiries for Medical Device Manufacturing License, please don’t hesitate to Reach Out to MedDev Experts. We are ready to provide comprehensive consulting services to help you navigate the regulatory requirements.
Good luck on your journey to contribute to the advancement of healthcare in India through medical device manufacturing license.
Stuck getting your MD 5 & MD 9 licenses?
Get expert guidance navigating India’s medical device licensing maze. MedDev Experts helps with:
MD 5 License: Secure state licenses for Class A & B devices. MD 9 License: Obtain manufacturing licenses from CDSCO.
MedDev Experts is one of the leading Medical Device Regulatory Consultant in India to provide expert guidance to get MD 5 License from State Licensing Authorities and MD 9 License from CDSCO.