FDA NDC Number Reservation Made Easy: A Comprehensive Guide to Drug Identification and Compliance
FDA NDC Number Reservation: An overview
Have you ever felt lost in the maze of FDA regulations when trying to bring your medical product to market? The FDA NDC Number reservation process can be particularly daunting, leaving many manufacturers scratching their heads. But don’t worry, you’re not alone in this struggle!
Table of Contents
ToggleImagine having a crystal-clear roadmap to navigate the FDA NDC Number reservation process effortlessly. Picture the peace of mind that comes with knowing your product is properly registered and compliant with all FDA requirements. That’s exactly what we’re here to help you achieve!
In this comprehensive guide, we’ll demystify the FDA NDC Number reservation process, breaking it down into easy-to-understand steps. We’ll explore which products are covered in the FDA NDC Number Directory, what information you need for reservation, and even provide a real-world example. Plus, we’ll reveal why MedDev Experts could be your secret weapon in mastering this crucial regulatory step. Ready to simplify your FDA compliance journey? Let’s dive in!
What is an FDA NDC Number?
An FDA NDC (National Drug Code) number is a unique, three-segment identifier assigned to human drugs in the United States. It serves as a universal product identifier for drugs, playing a crucial role in:
Facilitating drug listing and registration
Enabling efficient product tracking
Assisting in recall procedures
Supporting billing and reimbursement processes
Purpose of FDA NDC Number Reservation
Reserving an FDA NDC number is essential for manufacturers, repackagers, and private label distributors. This process:
- Ensures compliance with FDA regulations
- Allows for proper product identification in the market
- Facilitates smooth distribution and sales processes
All drug establishments must provide the Food and Drug Administration (FDA) with a list of all drugs they manufacture, prepare, propagate, compound, or process for commercial distribution. The FDA uses a unique, three-segment number called the National Drug Code (NDC) to identify and report drug products.
The NDC is a universal product identifier for human drugs. A registered establishment must update its drug listing data in June and December of each year. The listing information is to be submitted electronically.
Products Covered in the FDA NDC Number Directory
The FDA NDC Number Directory lists prescription drugs, OTC drugs, and insulin products that have been manufactured, prepared, propagated, compounded, or processed by registered establishments for commercial distribution in the US. The FDA NDC Directory covers a wide range of pharmaceutical and medical products:
Prescription drugs
Over-the-counter medications
Insulin products
Homeopathic drugs
Vaccines (except those regulated by CBER)
Products not Covered in the FDA NDC Number Directory
The NDC Directory does not list those products which are;
- If the products are not prescription drugs, OTC, or insulin products.
- If the products are no longer being marketed;
- If the manufacturer has not provided complete information about the product.
Information Required for FDA NDC Reservation
Product Trade Name or Catalog Name
Product names in the NDC Directory are limited to 100 characters. Some information may be omitted from product names, such as strength, USP/NF designations, and trademark symbols.
NDC Number
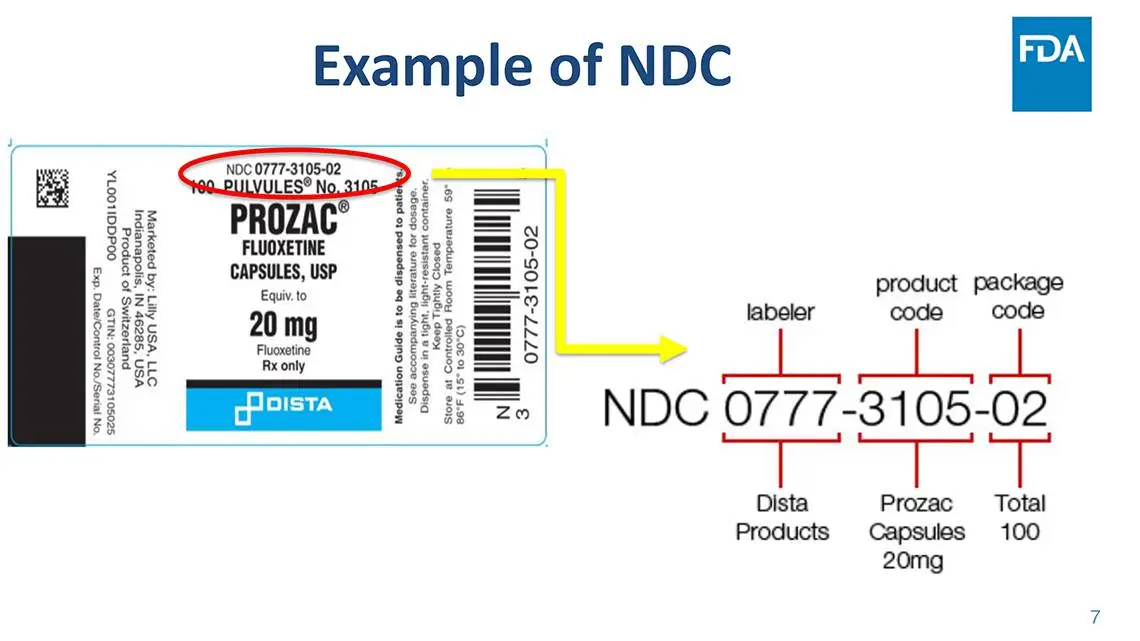
The FDA NDC (National Drug Code) is a unique 10-digit number assigned to each listed drug product. It identifies the labeler, product, and package size. The first segment is the labeler code assigned by the FDA, the second segment is the product code indicating strength and dosage form, and the third segment is the package code specifying package sizes and types.
Dosage Form
The dosage form refers to how a medication is prepared and presented for administration. It encompasses various physical formats such as tablets, capsules, liquids, creams, injections, and more.
Routes of Administration
Routes of administration are the different ways medications enter the body, including oral (by mouth), topical (on the skin), injectable (via injections), inhalation (breathed in), transdermal (through the skin), rectal (via the rectum), and nasal (through the nose) etc. The chosen route depends on the medication's properties, desired effects, patient's condition, and convenience. Each route has specific advantages in terms of absorption, onset of action, and distribution.
Active Ingredient(s)
The active ingredient refers to the key component or substance in a medication or product that produces its intended therapeutic effect.
Strength
Strength refers to the concentration or potency of the active ingredient in a medication or product. It indicates the amount of the active ingredient present per unit of dosage or volume.
Unit
Unit refers to the specific measurement or quantity in which a medication or substance is dosed or administered. It can vary depending on the context, such as milligrams (mg), micrograms (mcg), milliliters (mL), grams (g), or units specific to certain medications or substances. The unit of measurement is used to ensure accurate dosing and administration of the medication or substance.
Package Size and Type
Package size refers to the quantity or amount of a product contained within its packaging. It indicates the total volume, weight, or count of individual units in a package. The package size can vary depending on the product and its intended use. Package type refers to the physical form or presentation of the packaging itself. It includes various formats such as bottles, blister packs, tubes, vials, jars, sachets, boxes, or other specific packaging designs. The package type is chosen based on factors such as product protection, convenience, storage requirements, and regulatory guidelines.
Major Drug Class
Major drug classes refer to broad categories or groups of medications that share similar mechanisms of action or therapeutic uses. Examples of major drug classes include antibiotics, antihypertensives, antidiabetic agents, analgesics, antidepressants, anticoagulants, and antihistamines. These classes help categorize and classify medications based on their primary pharmacological effects and clinical indications.
FDA Approved Application Number
The NDA/ANDA number is assigned to a product approved by the FDA after assessing its safety, effectiveness, manufacturing processes, packaging, and labeling. This number indicates that the drug has met the necessary criteria for marketing approval.
Example of FDA NDC Number
Why choose MedDev Experts for NDC Number Reservation services?
Expertise and Comprehensive Solutions
MedDev Experts stands out as the top choice for NDC Number Reservation services due to our unparalleled expertise and comprehensive solutions. Our team of regulatory specialists offers:
- Complete regulatory compliance assistance
- Transparent and efficient service delivery
- Cost-effective rates with no hidden charges
- Free product classification evaluations
Streamlined Process and Ongoing Support
We simplify the complex NDC reservation process, handling everything from DUNS number acquisition to drug listing completion. Our commitment extends beyond initial registration:
Quick turnaround times (3-4 working days for drug listing)
Continued support until FDA website verification
Expert advice on drug categories and e-commerce regulations
Assistance with labeling and documentation requirements
Conclusion
In conclusion, the FDA NDC Number plays a crucial role in tracking and managing drug products. The NDC Directory serves as a valuable resource for healthcare providers, pharmacists, payers, and patients, providing essential information about drugs, including their names, dosage forms, strengths, routes of administration, active ingredients, and package details. By utilizing the NDC Directory, stakeholders can ensure the accuracy and safety of drug prescribing, dispensing, utilization tracking, and patient education.
If you are a manufacturer or exporter seeking guidance on NDC numbers and compliance, I encourage you to contact us. We have specialized knowledge and experience & can provide valuable assistance in navigating the complexities of the NDC system, ensuring accurate serving and updates of NDC numbers.
By leveraging our expertise, you can streamline your processes, maintain compliance, and establish a strong foundation for successful manufacturing and export of pharmaceutical products. Reach out to MedDev Experts today to benefit from their guidance and support.





Wow! Finally I got a website from where I be able to in fact
obtain useful information concerning my study and knowledge.