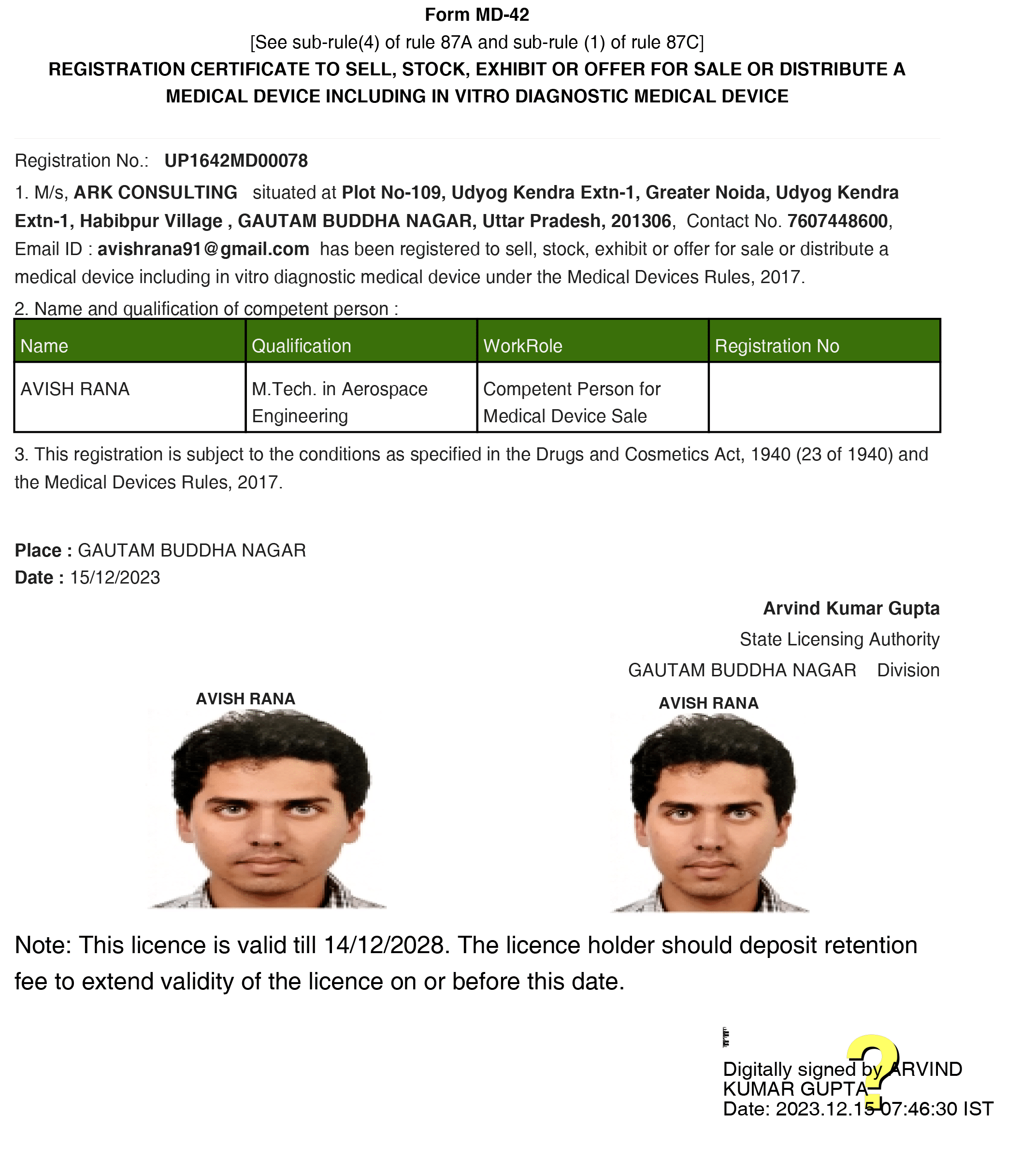
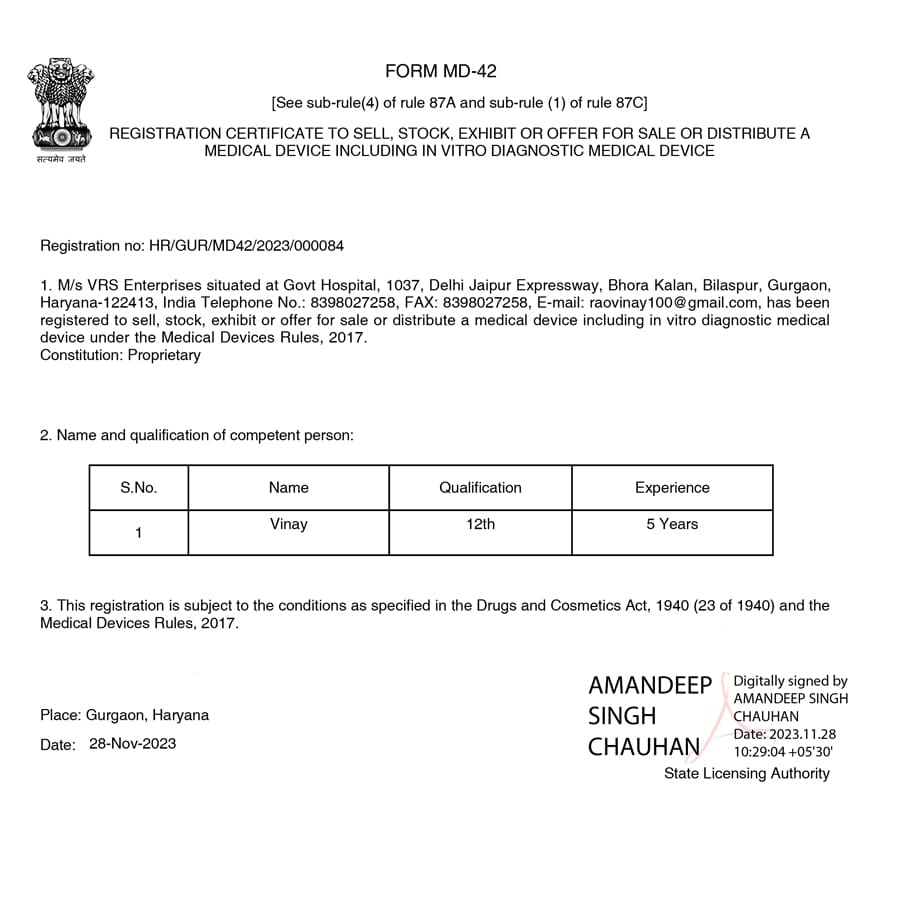
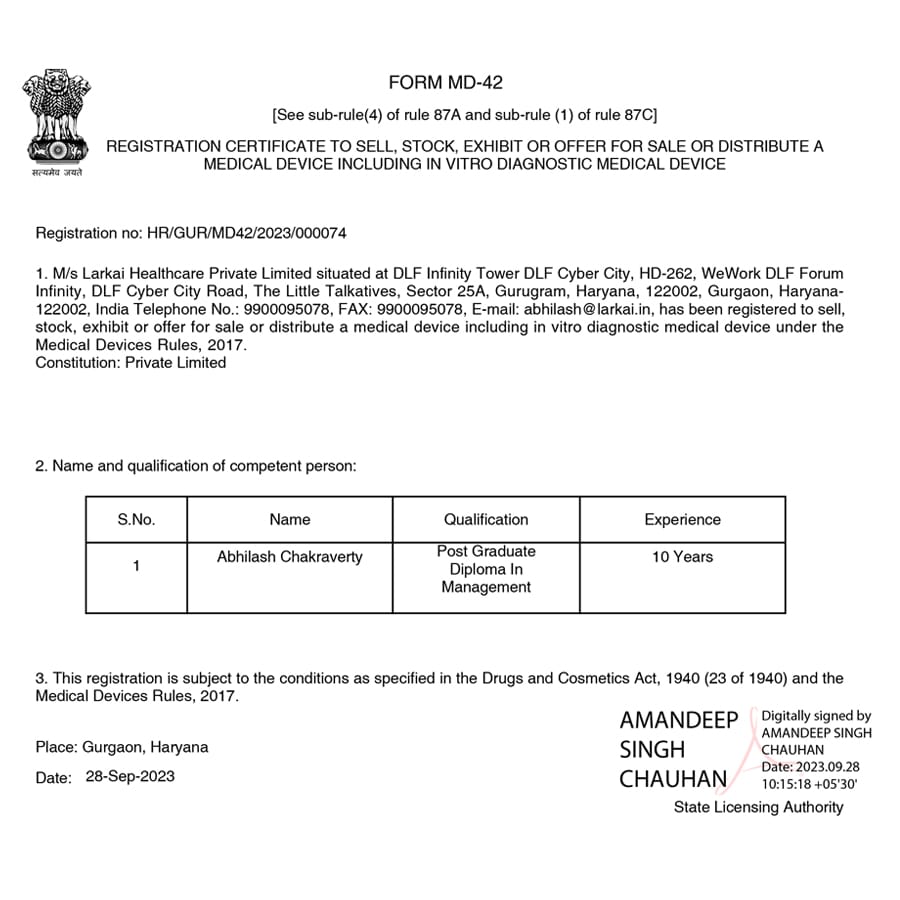
Do you need MD 42 Registration Certificate to sell medical devices and In-Vitro Diagnostic Devices in India?
The Ministry of Health and Family Welfare, Government of India has published the 5th Amendment of Medical Device Rule vide General Statutory Rule (G.S.R.) 754(E) on 30th September 2022. This Rule has made provision for Registration Certificate to sell, stock, exhibit, or offer for sale or distribute a medical device including In-Vitro diagnostic device.
It means if you are a retailer, wholesaler, or stockist of medical devices including IVDs, you must get a registration certificate from the state licensing authority on Form MD-42. The MD 42 Registration Certificate is mandatory from 30 September 2022.
In this article, we will discuss the Requirements, Processes, Conditions, Validity, and Renewal, of the MD 42 Registration Certificate.
- Receipt of fees
- Self-Certification of compliance to Good Distribution Practice.
- Identification proof of the applicant like Aadhar Card or PAN Card
- Rent Agreement or Sale Deed of the premises.
- Certificate and Experience Letter of the Competent Technical Staff
- A brief description on other activities carried out by applicant.
- An undertaking for compliance with the storage requirements of medical devices.
- Who holds a degree from a recognized University/Institution; or
- Who is a registered pharmacist; or
- Who has passed intermediate examination with one-year experience in dealing with sale of medical devices;
The Applicant must appoint a Competent Technical Staff to direct and supervise the sales activity of medical devices. The Competent Technical Staff must possess the above educational qualification and experience.
How to Get MD 42 Registration Certificate in India
- The applicant should fill the application form MD 41 on the online CDSCO Sugam Portal.
- Upload all the required documents.
- Pay the prescribed fee of Rs. 3000/-
- Submit the Application to the Licensing Authority.
- After scrutiny, the State Licensing Authority shall grant the MD 42 Registration Certificate.
- The Authority may conduct an inspection at our premises to verify all the compliance of the rule.
What Are the Terms & Conditions of MD 42 Registration Certification?
- The registration certificate must be displayed at a prominent place on the premises and visible to the public.
- The licensee must provide adequate space and proper storage conditions for the storage of medical devices.
- The licensee shall maintain the requisite temperature and lighting as per the requirements of such medical devices.
- The licensee shall purchase the devices only from the importer or licensed manufacturer or registered or licensed entity.
- The licensee shall maintain the sale and purchase records of all the devices and the records shall be made available for inspection by a Medical Device Officer.
- The licensee shall maintain all the records for a period of not less than two years from the last entry.
- The licensee shall maintain an inspection book in Form MD-43 to enable the Medical Devices Officer to record his observations and defects noticed
What is the fee for the MD 42 Registration Certificate for Medical Device?
The applicant has to pay ₹ 3,000/- for the Medical Device Registration Certificate MD-42 and the retention fee is ₹ 3,000/- which is to be paid every 5 years.
Validity and Renewal of the MD 42 Registration Certificate for Medical Device
Deadline to Obtain MD 42 License is 30 September 2022!
It is mandatory for all Retailer, Wholesaler, Stockiest and Importer to get MD 42 License to Sell & Stock Medical Devices in India
Conclusion
The Medical Device Registration Certificate MD-42 is a mandatory requirement for retailers, wholesalers, and stockiest of medical devices in India. The certificate is valid for five years and can be renewed by paying the retention fee. The process of obtaining the certificate is relatively simple and can be completed online.
If you are a retailer, wholesaler, or stockiest of medical devices in India, you must ensure that you have a valid MD-42 registration certificate. This will help to ensure that you are complying with the law and that you are selling safe and effective medical devices.
If you need any further assistance and guidance to get the MD 42 registration certificate, please contact our 24-Hour Customer Support No. +91-7807-91-4459. You can also WhatsApp your query to +91-7807-91-4459.
Don’t delay! Apply for your MD-42 registration certificate today and ensure that you are complying with the law. This will help to protect your customers and your business.
Get your MD 42 License within 3 to 4 working days in Delhi, Bengaluru, Chennai, Mumbai, Gurugram, Haryana, Noida, Uttar Pradesh, Chandigarh, Himachal Pradesh
Contact MedDev Experts Now!
Frequently Asked Questions for MD 42 Registration
Why Amazon, Flipkart, and other E-Commerce portals are asking for MD 42 License?
Amazon and Flipkart are asking for MD 42 License because it is a mandatory license to sell medical devices in India. MD 42 License is required for all medical devices that are to be imported, manufactured, or sold in India. This license is issued by the CDSCO.
Which medical devices are required to be registered under MD 42?
All medical devices including in-vitro diagnostic medical devices are required to be registered under MD 42. This includes a wide range of devices, such as surgical instruments, implants, diagnostic devices, and therapeutic devices.
How long does the MD 42 Registration process take?
The MD 42 Registration process can take up to 1 to 3 months.
What are the common reasons for rejection of MD 42 Registration applications?
The most common reasons for rejection of MD 42 Registration applications are:
- Incomplete application
- Insufficient supporting documentation
- Failure to meet the technical requirements of the Medical Devices Rules, 2017
Who is the consultant for MD 42 License?
MedDev Experts is the best consultant for MD 42 License. We have a team of experienced and qualified professionals who can assist you with every step of the MD 42 registration process. We have a proven track record of success in helping companies to obtain MD 42 Registration in a timely and efficient manner.
Where Can I get more information on MD 42 License?
Contact MedDev Experts to get a detail information on MD 42 License. We are regulatory consulting company specializing in the Medical Device Rules.
Who needs to obtain MD 42 Registration?
All wholesalers, retailers, and stockists of medical devices and in-vitro diagnostic medical devices in India must obtain MD 42 Registration.
Can I modify my MD 42 License after it's issued?
Yes, you can apply for post-approval changes of your MD 42 License as needed.
Can I transfer my MD 42 License to another entity?
No, MD 42 License is non-transferable. It is specific to the entity that applied for it.
How can I check the status of my MD 42 License application?
You can check the status of your application online through the CDSCO SUGAM portal.
Can I sell imported medical devices without MD 42 License?
No, you must obtain MD 42 License to sell imported medical devices.
Can I apply for multiple MD 42 Registrations for different product categories?
No, MD 42 Registration is issued for all medical devices and in-vitro diagnostic medical devices.
Can I sell medical devices online without MD 42 Registration?
No, you can’t sell medical devices online without MD 42 Registration.
We MD 42 Licenses Achieved by Us


Let’s Get Started on Your Medical Device Licensing Journey










