The Best CDSCO Medical Device Consultant in Ranchi, Jharkhand
Optimize Simplification of Your CDSCO Medical Device Licensing & Registration
Your search for the finest CDSCO Medical Device Consultant in Ranchi, Jharkhand concludes here. MedDev Experts, your trusted partner in obtaining CDSCO Medical Device Licenses, sets the benchmark with an impeccable reputation and a remarkable history of accomplishments. Our unwavering commitment is centered on delivering exceptional services to our esteemed clients.
WELCOME TO
Get ready for a seamless journey as MedDev Experts propels you forward into the intricate realm of medical device licensing and registration. With our seasoned team of experts as your steadfast guides, we ensure meticulous compliance with all requirements, transforming the process of acquiring essential licenses into an efficient and streamlined experience.
We offer effortless registration for medical devices with the CDSCO and State FDA of Jharkhand, CDSCO Medical Device Import License for Indian market entry, secure wholesale licenses for selling, stocking, exhibiting, or distributing medical devices. Propel your organization to new heights with ISO certification, surpass industry standards with GMP compliance, conquer new horizons through FDA product listing, and catapult your product into the US market with 510(k) submission to the FDA.
Simplify your CDSCO medical device licensing and registration journey today by partnering with MedDev Experts in Ranchi, Jharkhand. We are the epitome of trust and reliability in CDSCO medical device consulting. Contact us now to embark on a path of unrivaled success.
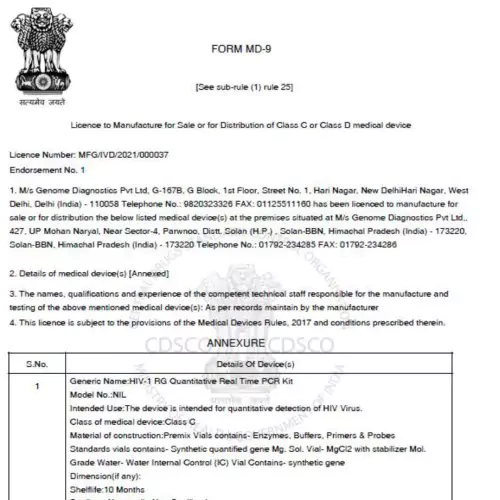
- Medical Device Manufacturing License: Register your medical devices with the CDSCO and State FDA of Uttar Pradesh effortlessly.
- Medical Device Import License: Obtain the CDSCO Medical Device Import License required for importing medical devices into India.
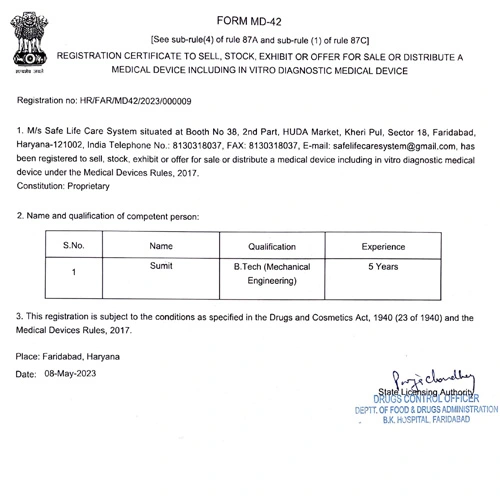
- Wholesale License for Medical Device: Secure the registration certificate to sell, stock, exhibit, or distribute medical devices.
- ISO Certification: Attain ISO 9001, ISO 13485, SA 8000, and other international quality standards with our assistance.
- GMP Compliance: Ensure your manufacturing process adheres to Good Manufacturing Practices (GMP) with our expert guidance.
- FDA Product Listing: List your medical device on the US FDA, facilitating exports to the US market.
- 510(k) Submission: Submit the Premarket Submission to the FDA for market placement in the US.
Leading CDSCO Medical Device Consultant in Ranchi, Jharkhand
Successful Licensing Partnerships: Companies We’ve Empowered.

Are you ready to start your medical device licensing journey? Our team of experts can help you navigate the complex regulatory process and get your product to market quickly and efficiently.




