Best CDSCO Medical Device Consultant in Haridwar, Uttarakhand
Achieve CDSCO Medical Device Licensing & Registration with Ease
MedDev Experts is a CDSCO Medical Device Consultants in Haridwar, Uttarakhand helping medical device manufacturers, importers, and wholesalers obtain CDSCO Medical Device Licenses in the Jammu & Kashmir. MedDev Experts is a trusted name in the CDSCO medical device consultancy industry with a proven track record of success.
WELCOME TO
We simplify the medical device licensing and registration process for manufacturers, importers, and wholesalers. Our team of experienced professionals will guide you through every step, from understanding the requirements to obtaining the necessary licenses.
- Medical Device Manufacturing License: Register your medical devices with the CDSCO and State FDA of Uttar Pradesh effortlessly.
- Medical Device Import License: Obtain the CDSCO Medical Device Import License required for importing medical devices into India.
- Wholesale License for Medical Device: Secure the registration certificate to sell, stock, exhibit, or distribute medical devices.
- ISO Certification: Attain ISO 9001, ISO 13485, SA 8000, and other international quality standards with our assistance.
- GMP Compliance: Ensure your manufacturing process adheres to Good Manufacturing Practices (GMP) with our expert guidance.
- FDA Product Listing: List your medical device on the US FDA, facilitating exports to the US market.
- 510(k) Submission: Submit the Premarket Submission to the FDA for market placement in the US.
Don’t let the complexities of CDSCO medical device licensing and registration overwhelm you. MedDev Experts in Haridwar, Uttarakhand is your trusted partner for simplified solutions. Contact us now and pave the way for unparalleled success.
Successful Licensing Partnerships: Companies We’ve Empowered.
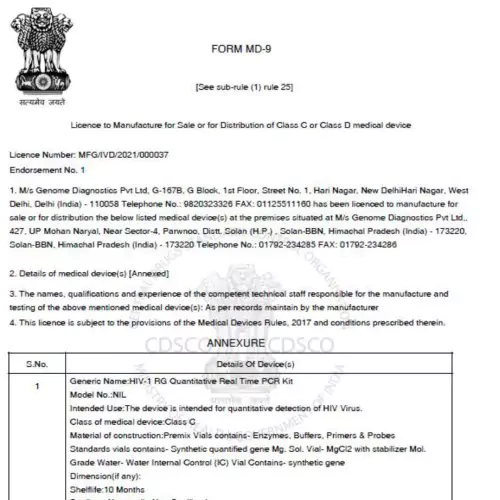

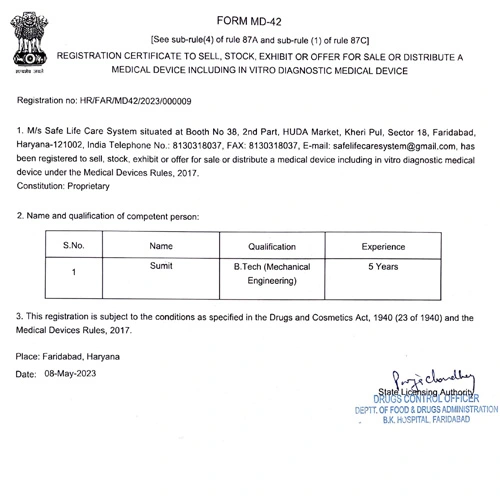
Are you ready to start your medical device licensing journey? Our team of experts can help you navigate the complex regulatory process and get your product to market quickly and efficiently.




