Unique Device Identification (UDI): How to Comply with FDA Guidelines?
How to Comply with the UDI Rules of FDA?
The implementation of the Unique Device Identification (UDI) system brings significant advancements to the medical device industry. The FDA’s UDI Rule mandates the inclusion of a unique device identifier on medical device labels, facilitating enhanced traceability, post-market safety activities, and monitoring by regulatory authorities.
Table of Contents
ToggleBy understanding the basics of UDI and its requirements, manufacturers and stakeholders can navigate the regulatory landscape more effectively and contribute to improved patient safety and device management.
Introduction to Unique Device Identification (UDI)
The unique device identification system adequately identifies medical devices sold in the market from manufacturing through distribution to patient use. The FDA UDI Rule requires the labeler to include a unique device identifier (UDI) on the labels and packages of the devices, unless there are specific exceptions or alternatives allowed by the rule.
If a device is meant to be used multiple times and needs to be reprocessed before each use, the UDI must also be directly marked on the device itself. The labeler must submit device information to the Global Unique Device Identification Database (GUDID). This database serves as a centralized repository for UDI-related data.
In summary, the UDI Rule mandates manufacturers to label their devices with a unique identifier and share relevant information about the devices in the GUDID.
Who is a Labeler?
A labeler refers to any individual or entity that is responsible for applying or modifying a label on a device, with the intention of commercially distributing the device without further label changes. The labeler is typically the device manufacturer, but it can also include other entities such as specification developers, single-use device re-processors, convenience kit assemblers, re-packagers, or re-labelers.
A Closer Look at the Unique Device Identification (UDI) Format
The UDI is a distinctive code made up of either numeric or alphanumeric characters, which typically comprises two parts: the Device Identifier (DI) and the Production Identifier (PI).
The Device Identifier (DI)
The device identifier (DI) is a required component of the Unique Device Identifier (UDI). It serves as a fixed portion of the UDI and is used to identify both the labeler (typically the manufacturer) and the specific version or model of a device. The DI helps ensure the unique identification and traceability of medical devices throughout their lifecycle.
The Production Identifier (PI)
The production identifier (PI) is a component of the Unique Device Identifier (UDI) that is conditional and variable. It is used to provide additional information on the label of a device. The PI can include one or more of the following details:
- Lot or batch number: This identifies the specific lot or batch within which a device was manufactured. It helps track and trace devices in case of any quality or safety issues.
- Serial number: This identifies the unique serial number of an individual device. It allows for precise identification and tracking of a specific unit throughout its lifecycle
- Date of manufacture: This signifies the specific date when a device was manufactured. It provides information about the production timeline and can be useful for quality control and inventory management purposes.
- Expiration date: This indicates the date when a specific device is expected to expire and should no longer be used. It is particularly relevant for devices with limited shelf life or those that have expiration date requirements for safety and efficacy.
- Distinct identification code for HCT/P: This pertains to human cell, tissue, or cellular and tissue-based products (HCT/P) regulated as devices. These products require a distinct identification code as per the requirements outlined in §1271.290(c).
Presentation of Unique Device Identifier (UDI)
Under the FDA UDI Rule, the device labeler is required to provide the Unique Device Identifier (UDI) in two forms on the labels and packages of medical devices:
- Easily Readable Plain-Text: The UDI must be presented in a format that is easily readable by humans. This typically means using clear, legible text that can be easily understood without the need for any specialized equipment.
- Machine-Readable Form: In addition to the plain-text form, the UDI must also be encoded in a machine-readable format that utilizes automatic identification and data capture (AIDC) technology. This enables the UDI to be scanned or captured electronically using barcode scanners, RFID (Radio Frequency Identification) readers, or other similar devices.
By providing the UDI in both easily readable plain-text and machine-readable form, the device labeler ensures that the UDI is accessible and usable by both humans and automated systems, promoting efficient tracking and identification of medical devices.
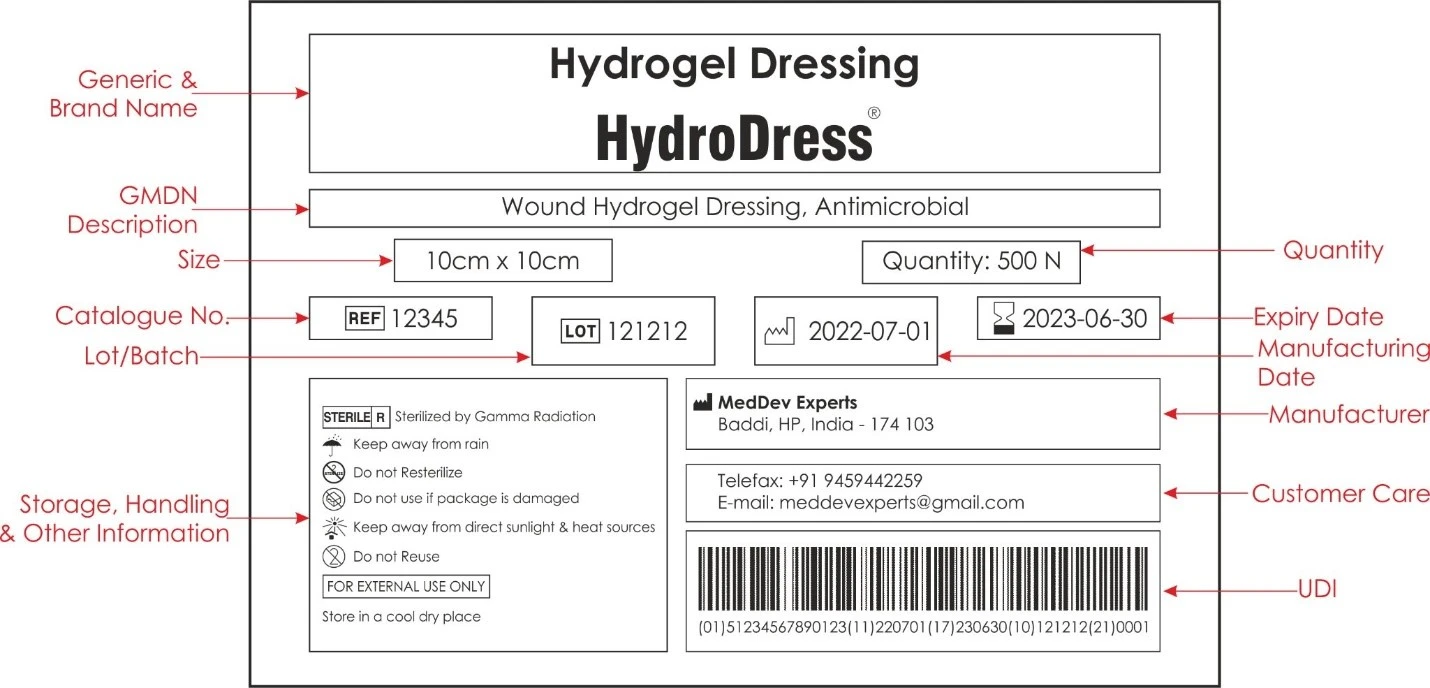
Example of a Unique Device Identification (UDI)
The following UDI Label example is solely for illustrative purposes.
The following UDI Label example is solely for illustrative purposes.
Unique Device Identification (UDI) Issuing Organization
Deadlines for Compliance with UDI Rule
The FDA has issued a reminder to the device industry and UDI program stakeholders to comply with the UDI Rule before September 24, 2023. Starting from September 24, 2023, devices must comply with all UDI requirements on labels.
Submitting Information to the Global Unique Device Identification Database (GUDID)
Device labelers have an obligation to submit information to the GUDID, which is administered by the FDA. GUDID stores a standardized set of fundamental identifying elements for each device that has a Unique Device Identifier (UDI). However, it’s important to note that GUDID solely includes the device identifier (DI), which acts as the primary key for retrieving device information from the database. The production identifier (PI), on the other hand, is not included in GUDID.
Benefits of Unique Device Identification (UDI)
The fully implemented Unique Device Identification (UDI) System offers a range of benefits to multiple stakeholders. It enables more accurate and efficient reporting of adverse events, leading to quicker identification and resolution of device issues. Medical errors are reduced as healthcare professionals can rapidly identify devices and access crucial information.
The UDI System enhances device analysis, supporting post-market surveillance, facilitating device approvals, and improving recall management. It also establishes a secure global distribution chain, combating counterfeiting and preparing for emergencies.
Implementation Challenges
Implementing UDI on device labels and packaging requires careful consideration of space limitations, readability, and design consistency. Ensuring that labels and packages meet regulatory requirements while maintaining aesthetic appeal can be a challenge.
Exemption from Unique Device Identification (UDI)
The followings are the exemption from the UDI Rule. Please refer to the UDI Rule for more details.
- Class I devices that FDA has exempted from Good Manufacturing Practices
- Individual single-use devices, all of a single version or model, that are distributed together in a single device package
- Custom-made device
- Device intended for export from the United States
Conclusion
In conclusion, the introduction of the UDI system revolutionizes the identification and tracking of medical devices. The FDA’s UDI Rule establishes clear guidelines for labelers to incorporate unique device identifiers on labels and packages, with necessary submissions to the Global Unique Device Identification Database (GUDID).
Thank you for taking the time to read our blog on the FDA UDI Rule. We hope that this information has provided you with valuable insights into the importance and implementation of the unique device identification system. If you have any further questions or require additional assistance, please don’t hesitate to Contact Us. Thank you again, and we appreciate your support!



A detailed blog about the UDI Basics. Great job!!!