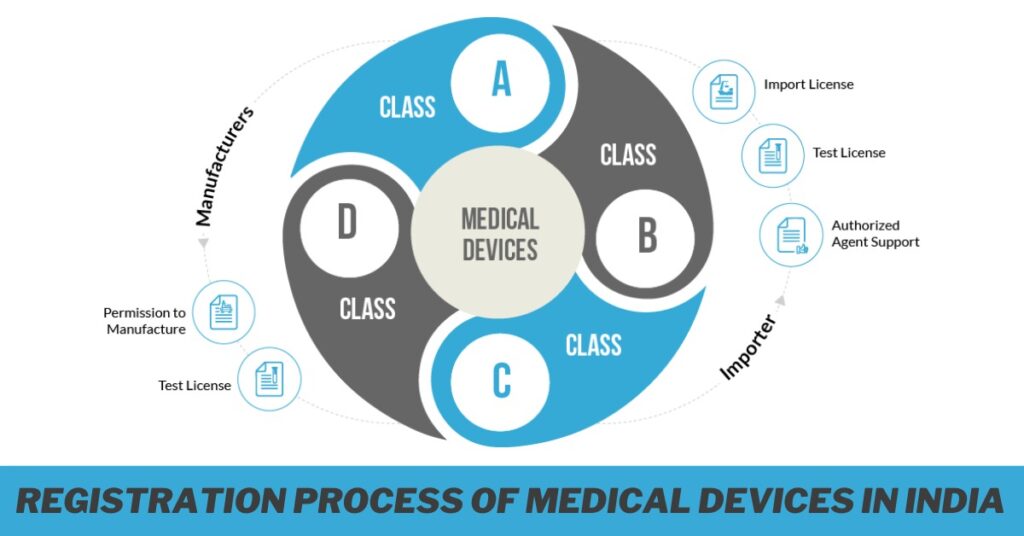
Introduction to Medical Device Import License 2024
The manner of importing clinical devices often involves acquiring a license, which calls for adherence to diverse criminal regulations. This article objectives to explore the complexities of criminal charges related to obtaining such licenses and offers techniques for handling these costs successfully. Importance of Legal Compliance Compliance with regulatory frameworks is paramount within the medical […]
Introduction to Medical Device Import License 2024 Read More »